Abstract
Traditional methods for chimeric antigen receptor (CAR) T manufacturing utilize viral vectors, ex vivo activation and expansion of T cells to achieve clinically relevant cell numbers, which leads to an exhausted T cell phenotype, high manufacturing costs, and treatment delays. The UltraCAR-T platform is designed to overcome these limitations using our advanced non-viral gene delivery system and a rapid, overnight manufacturing process (Blood 2019 134 (Supplement_1):2660; Blood 2020 136 (Supplement 1):17); Cancer Research 2020 80 (16Suppl):6593). UltraCAR-T cells, which express antigen specific CAR, membrane-bound IL-15 (mbIL15), and kill switch genes, are manufactured at the medical center's cGMP facility using autologous T cells and administered back to the patient only one day after gene transfer. UltraCAR-T cells are currently under clinical investigation for hematological (NCT03927261) and solid tumors (NCT03907527).
Here we describe the advancement of the UltraCAR-T platform to address the inhibitory tumor microenvironment by incorporating intrinsic checkpoint blockade without the need for complex and expensive gene editing techniques. PRGN-3007, based on the next generation of the UltraCAR-T platform, is engineered to simultaneously express CAR for targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1), which is overexpressed on many hematological and solid tumors; mbIL15 for enhanced in vivo expansion and persistence; kill switch for improved safety profile; and a novel mechanism for the intrinsic blockade of PD-1 gene expression. This approach of intrinsic blockade of PD-1 expression, only on UltraCAR-T cells, is aimed to avoid systemic toxicity and high cost of checkpoint inhibitors by eliminating the need for combination treatment. PRGN-3007 is manufactured using the already established rapid and streamlined UltraCAR-T manufacturing process.
PRGN-3007 was generated using multiple healthy donor T cells using multi-cistronic non-viral vector and the overnight manufacturing process. The co-expression of CAR, mbIL15 and kill switch transgenes was confirmed by flow cytometry, western blotting, and qPCR. Furthermore, PRGN-3007 showed significant reduction in PD-1 expression on UltraCAR-T cells compared to ROR1 CAR-T cells lacking PD-1 blockade (Control ROR1 CAR-T). The downregulation of PD-1 expression on PRGN-3007 resulted in enhanced ROR1-specific cytotoxicity and release of inflammatory cytokines upon co-culture with various ROR1 + PD-L1 + hematological and solid tumor cells compared to Control ROR1 CAR-T, especially at low effector to target cell ratios. Single-cell cytokine proteomics showed that the downregulation of PD-1 expression on PRGN-3007 resulted in a significantly higher number of polyfunctional CAR-T cells compared to Control ROR1 CAR-T. Expression of mbIL15 on UltraCAR-T, with or without downregulation of PD-1 expression, resulted in robust expansion in presence of ROR1 antigen, lack of autonomous expansion in absence of ROR1, and durable persistence even in absence of exogenous cytokines in vitro. Furthermore, PRGN-3007 was selectively and effectively eliminated by the kill switch activator antibody treatment.
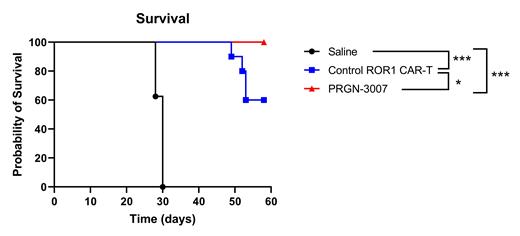
A single administration of PRGN-3007, only one day after gene transfer, effectively reduced tumor burden and significantly improved overall survival (p<0.05) of tumor bearing mice compared to Control ROR1 CAR-T in an aggressive xenograft model of mantle cell lymphoma (Figure). Blood analyses demonstrated sustained downregulation of PD-1 expression, rapid expansion, long-term persistence, and a predominant central memory phenotype of PRGN-3007 in tumor bearing mice.
In summary, these preclinical data highlight the overall safety and improved efficacy of incorporating intrinsic downregulation of PD-1 expression on UltraCAR-T cells using non-viral gene delivery and the established rapid, decentralized manufacturing process. These data provide a strong rationale for the evaluation of PRGN-3007 for the treatment of ROR1 + malignancies.
Figure: Overall survival in in an established model of mantle cell lymphoma in NSG mice. Tumor cells were engrafted in mice on Day 0 and treatments were administered on Day 8. Data shown is from 8 mice/group at the start of the study. * p<0.05, ***p<0.001; log rank test.
Chan: Precigen, Inc: Current Employment, Current equity holder in publicly-traded company. Scott: Precigen, Inc: Current Employment. Du: Precigen, Inc: Current Employment. Bolinger: Precigen, Inc: Current Employment. Poortman: Precigen, Inc: Current Employment. Shepard: Precigen, Inc: Current Employment. Koenitzer: Precigen, Inc: Current Employment. Govekung: Precigen, Inc: Current Employment. Sailor: Precigen, Inc: Current Employment. Johnson: Precigen, Inc: Current Employment. Plummer: Precigen, Inc: Current Employment. Zilko: Precigen, Inc: Current Employment. Dutta: Precigen, Inc: Current Employment. Kunchithapautham: Precigen, Inc: Current Employment. Athwal: Precigen, Inc: Current Employment. Klocke: Precigen, Inc: Current Employment. Zinser: Precigen, Inc: Current Employment. Abdeladhim: Precigen, Inc: Current Employment. Ahmad: Precigen, Inc: Current Employment; Kite, A Gilead Company: Ended employment in the past 24 months. Metenou: Precigen, Inc: Current Employment. Semnani: Precigen, Inc: Current Employment. Brough: Precigen, Inc: Current Employment, Current equity holder in publicly-traded company. Shah: Precigen: Current Employment, Current equity holder in publicly-traded company. Sabzevari: Precigen: Current Employment, Current equity holder in publicly-traded company; Kinnate BioPharma: Membership on an entity's Board of Directors or advisory committees; Compass Therapeutics: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal